Abstract
Background: Small-molecule FLT3 tyrosine kinase inhibitors (TKIs) have improved remission rates in relapsed/refractory AML driven by FLT3 internal tandem duplication (ITD) mutations, although nearly all responders eventually relapse. Acquired clinical resistance to type II inhibitors such as quizartinib and sorafenib has largely been due to kinase domain (KD) mutations that destabilize drug binding. Early inhibitors, limited by short half-life and fluctuating plasma levels, were also only able to transiently inhibit FLT3. FF-10101 is a first-in-class, type I inhibitor that covalently binds FLT3 irreversibly, suggesting its activity should be limited by the half-life of FLT3 itself. In a profiling assay of 216 human recombinant kinases FF-10101 showed selectivity for wild type FLT3 and FLT3 D835Y. It also demonstrated potent activity against FLT3-ITD AML cell lines harboring TKI-resistant D835Y and Y842C/H mutations. Here, we report in vitro testing of FF-10101 against 19 additional FLT3 TKI-resistant mutations, as well as six cysteine-to-serine mutations within the KD that could impact covalent drug binding. Profiling of this activity is relevant as FF-10101 enters single-agent phase 1/2a clinical trial in relapsed/refractory FLT3 mutant AML.
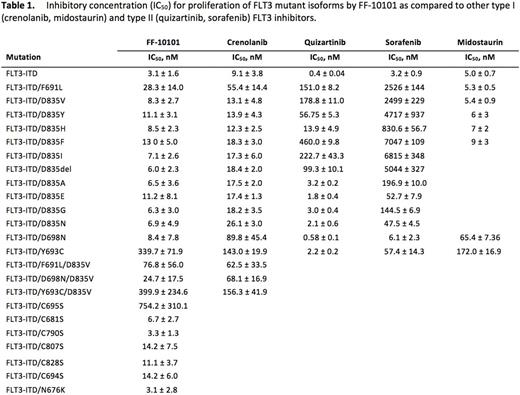
Results: FF-10101 was profiled in an in vitro viability assay against murine BaF3 and human AML cell lines harboring a spectrum of mutations. FF-10101 inhibited the proliferation of FLT3-driven Molm14 and MV4;11 cells (IC50 4.1 and 1.1 nM, respectively). BaF3 FLT3-ITD cells were comparably inhibited (IC50 3.1 ± 1.6 nM), while growth of BCR-ABL-driven K562 cells were unaffected. To confirm covalent binding of FF-10101, we then analyzed its activity against all cysteine residues in the FLT3 KD. Every C->S substitution except C695S remained sensitive to FF-10101, confirming C695 as the FF-10101 binding site and suggesting C695S may impart clinical resistance to FF-10101. We next assessed 10 BaF3 FLT3-ITD cell lines harboring mutations at D835 and the gatekeeper F691, given discovery of resistance mutations at these sites in patients treated with AC220 and sorafenib. FF-10101 inhibited F691L and all substitutions/deletions at the D835 residue. N676K, a mutation found in a patient at relapse on midostaurin, was also potently inhibited. Notably, F691L previously exhibited relative resistance to crenolanib, a reversible, type I FLT3 inhibitor. Beyond clinically described mutants, we also tested FF-10101 against six mutations previously identified in mutagenesis screening assays. A D698N mutation found in separate crenolanib and PLX3397 mutagenesis assays demonstrated relative resistance to crenolanib but was sensitive to FF-10101 (IC50 8.4 ± 7.8 nM), as were four D839 amino acid substitutions identified in the PLX3397 mutagenesis assay alone. Alternatively, a Y693C mutation found in a crenolanib mutagenesis screen remained relatively insensitive to FF-10101. Finally, two resistance mutations co-occurring in the same KD are well-described in chronic myelogenous leukemia (CML) patients treated with sequential TKI therapy. While not yet found in AML patients treated similarly, we hypothesize that analogous compound mutations could emerge. As such, we tested FF-10101 against BaF3 FLT3-ITD cells containing three compound mutations. Unlike crenolanib, FF-10101 potently inhibited D698N/D835V, but F691L/D835V and Y693C/D835V each exhibited relatively moderate resistance to both drugs. Analysis of additional activation loop (AL) mutations (N831H/K, R845G, G846R, Y842S) and biochemical studies of FLT3 phosphorylation and downstream effector pathways in response to FF10101 is ongoing and will be presented.
Conclusions: FF-10101 is a type I, irreversible FLT3 inhibitor that has demonstrated potent activity against all mutations associated with clinical resistance to FLT3 TKIs. Expanded profiling shows anti-proliferative activity against nearly all mutations associated with FLT3 TKI resistance except for C695S (the site of FF-10101 binding) and the crenolanib-resistant Y693C mutation, which has yet to be associated with clinical TKI resistance. These data suggest FF-10101 has potential to be clinically active against the majority of FLT3 TKI resistant KD mutations. FF-10101 is currently beginning phase I/2a clinical assessment as a single agent in patients with relapsed/refractory AML harboring a FLT3 mutation.
Smith: Fujifilm: Research Funding; Astellas: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal